Gary Taubes argues that energy imbalance (calories in > out) does not cause obesity; rather, energy imbalance is a result of the fattening process. In this post, I evaluate this claim, which boils down to the question of which side of the energy balance equation is driving the other. A version of this debate has been happening within the obesity research community, including my corner of it, for decades. The truth is that while fat mass can clearly be changed by altering energy balance, and energy imbalance is required for obesity to occur, we don’t know for sure which side of the equation drives the development of common obesity. Current evidence suggests that energy imbalance probably drives obesity development, but the body fat regulatory system may drive energy imbalance and contribute to obesity development as well. Regardless of this, the evidence suggests that the primary body system in charge of the energy balance equation as a whole is probably the brain, not insulin or fat tissue.
I recently had a spirited debate on Twitter with journalist and low-carb diet advocate Gary Taubes about the energy balance equation. The debate relates to a long-standing difference of opinion about the implications and usefulness of the equation. Taubes’s arguments about energy balance have been persuasive to the general public, and a few researchers, so I think it’s worthwhile to address them. This was also an opportunity for me to clarify my own thinking.
What is the energy balance equation, and what is the claim about it?
The energy balance equation states that changes in the amount of energy in the body are equal to the amount of energy entering the body, minus the amount leaving:
Change in body energy = Energy in – Energy out
This equation is an expression of the first law of thermodynamics, it’s simple arithmetic, and it’s not in question. Since fat tissue is by far the most capacious and most modifiable energy storage site in the body, substantial changes in the energy content of the body are represented by changes in body fat mass.
The body of a person with mild obesity contains about twice as much energy as the body of a lean person of the same sex and height, and this difference in body energy content is accounted for almost exclusively (~92%) by differences in fat mass.* It is of obvious interest to understand how this extra energy arrived in the body.
On many occasions, including in his new book The Case for Keto, Taubes has expressed a dim view of how he believes the energy balance equation is interpreted by researchers:
As sceptics pointed out at the time, though, the energy balance notion has an obvious flaw: it is tautological. If we get fatter (more massive), we have to take in more calories than we expend— that’s what the laws of thermodynamics dictate—and so we must be overeating during this fattening process. But this tells us nothing about cause. Here’s the circular logic:
Why do we get fat? Because we overeat.
How do we know we’re overeating? Because we’re getting fatter.
And why are we getting fatter? Because we’re overeating.
And so it goes, round and round
Gary Taubes. BMJ 346:16-17. 2013.
The key claims in this passage are that the energy balance equation tells us nothing about what causes obesity (“this tells us nothing about cause”), and assuming that energy imbalance causes obesity because of the energy balance equation is illogical circular reasoning (“here’s the circular logic”).
The first thing that stands out to me is that I can’t recall having encountered in the scientific literature a circular argument like the one Taubes makes. I certainly don’t recognize it as a pervasive or fundamental way of thinking in my field. The common argument is that the energy balance equation implies that energy in > out causes obesity. That may be debatable but it isn’t circular. Taubes’s version is circular because he tacks on a series of self-referencing assertions that aren’t part of the original argument. In his telling, researchers answer questions about obesity by making assumptions that are based on one another.
Fortunately, researchers have a better way of answering questions than making circular assumptions: experimental evidence. For example, we don’t have to assume anything about energy intake– we can measure it. That’s how we know it’s typically higher in people with obesity. See this graph of accurately measured energy intake graphed against body mass index of 15 men of widely varying body fat levels.**
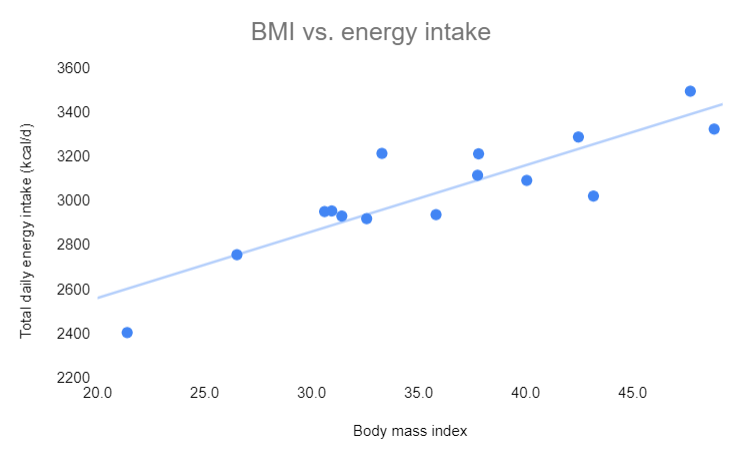
Likewise, we don’t have to assume anything about the impact of increasing energy intake on body fatness– we can measure it. That’s how we know increasing energy intake increases body fatness, regardless of whether the excess energy comes from carbohydrate or fat.
In the end, I don’t know what to make of Taubes’s circular argument. It’s obviously illogical but I don’t recognize it from the scientific literature, and it ignores quite a bit of relevant scientific evidence. I’m not sure who it’s arguing against. Maybe I’m missing something?
Nevertheless, I think there’s a logical idea in the passage that gets at an interesting question. So I’m going to steel-man it, which means to present the strongest, most charitable version of it that I can. Here goes:
- People often focus on the right side of the energy balance equation (energy in/out) as driving the left side (change in body energy). “She ate more, therefore she gained fat”.
- However, the equation itself doesn’t imply directionality.
- It’s possible that this is causally flipped in the development of common obesity, where the original cause is a biological process that drives fat gain, and energy imbalance is simply a tool serving that process. “Her body was ‘trying’ to gain fat, and this process caused her to eat more and/or reduced her metabolic rate”.
- If the latter scenario is true, it would be more appropriate to apply the word “cause” to the biological process rather than energy imbalance, since the former is ultimately driving the latter.
An analogy Taubes uses frequently is that of growth. In the case of a child becoming an adult, wouldn’t we say that the growth process is causing the child to eat more, rather than eating more causing the child to grow?
The argument is logical, but that doesn’t necessarily mean it’s correct in the context of common obesity. Let’s evaluate it.
Energy imbalance is causal, but that doesn’t necessarily mean it’s in charge
As mentioned previously, the body of a person with mild obesity contains about twice as much energy as the body of a lean person, and nearly all (~92%) of the excess energy is in fat tissue.*
According to physics and simple arithmetic, as expressed by the energy balance equation, this energy accumulation must result from energy imbalance (energy in > out). There is no other possible way for the extra energy to arrive in the body. Energy imbalance is required for the state of common obesity to develop. It is therefore causal, as opposed to just being an innocent bystander. That said, I acknowledge that there is an important semantic issue around how we use the word “cause”. I’ll come back to that.
Energy imbalance is also causal in the case of a child growing, because an adult’s body contains more energy than a child’s body. The normal transition from child to adult cannot occur without energy imbalance. But growth isn’t a very good analogy for obesity. One reason is that in obesity, excess energy in the body is the condition. The difference in the energy content of a lean body vs. an obese body is nearly all accounted for by the difference in fat tissue mass, which is the definition of obesity. That isn’t the case in development. A second reason the analogy fails is that increasing a well-nourished person’s calorie intake causes them to gain body fat, while increasing a well-nourished child’s calorie intake does not cause them to develop into an adult. Similarly, reducing calorie intake causes body fat loss but doesn’t cause an adult to regress back to a child. I won’t consider this analogy further.
I think the best way to think about obesity, and everything else, is as a causal chain (or causal web if you want to be fancy). Everything is caused by something, so whatever our variable of interest (e.g., body fatness), it was caused by one or more upstream factors, which in turn were caused by something else, ad infinitum. Each cause is a link in the chain. No matter what your model of obesity is, in order to explain the development of an obese body, energy imbalance has to be a link in the model’s causal chain. If it isn’t, you’ve run afoul of physics and arithmetic.
That said, I do think Taubes’s argument has some logic, and it comes down to the question of what is ultimately controlling the system. In a typical causal chain, there are some links that actively determine the system’s output, and other links that just transmit the signal. Another way of thinking about this is that some links “give orders” and other links just “take orders”. The logical piece of Taubes’s argument is that the energy balance equation itself doesn’t imply which side is giving orders, and which is taking orders.
Consider this example. The speed of a car is determined (in large part) by the amount of force its tires exert on the road. Tires cause a car to move forward. Yet if we want to understand why some cars tend to drive faster than others on a local highway, studying their tires won’t provide any insight into that: the tires are just taking orders from upstream links in the causal chain (axle, transmission, engine, accelerator, etc.). To understand why some cars drive faster than others, we have to focus on the part of the system that’s giving orders, which is the person behind the wheel.
But what do we apply the word “cause” to? This might seem like splitting semantic hairs, but I think it’s actually at the heart of this debate. Is every link in the causal chain a “cause”? Or does a link have to give orders to be a “cause”? Taubes appears to use the latter definition, others often use the former definition, and this causes the two sides to talk past one another. I believe this semantic miscommunication explains a substantial part of the disagreement.
I’ll give an example to make this more concrete. Here is a passage from a post on Taubes’s website, in which he quotes from a review paper written by my scientific mentor Mike Schwartz:
“Obesity, by definition, results from ingesting calories in excess of ongoing requirements,” Schwartz and his co-author write, and I’m arguing that any researcher who makes this statement for any reason other than to make the point that it’s meaningless and misleading is living and working in an energy balance paradigm — calories-in, calories-out.
Taubes interprets this passage as implying that energy imbalance is giving orders, leading him to heap contempt on it. Yet if one reads the paper, it’s about the biological regulation of fat mass and energy balance. Furthermore, the paper explicitly discusses the question of whether energy imbalance leads or follows in the development of common obesity, and concludes that the question is unresolved***.
Clearly, Taubes’s interpretation of the passage is not what the authors intended. The reason for the miscommunication is that the paper is making the uncontroversial observation that energy imbalance is a link in the causal chain of obesity, while Taubes interprets it as a statement about which side of the equation is giving orders. Taubes does this a lot, but in fairness I think the research community is often guilty of thinking/communicating less than optimally on this issue. In this particular case the context makes the intention clear, but that isn’t always true.
Extending the car analogy to obesity, the steel-man version of Taubes’s argument would state that yes, energy imbalance is in the causal chain leading to obesity, but it’s just taking orders. The left side of the energy balance equation– a biological process driving fat mass expansion– is what’s giving orders. That’s the theory, but is it correct?
What’s giving orders, and what’s taking them?
The first thing I’d like to point out is that the left and right sides of the energy balance equation could both be giving orders, and both be taking orders. The two possibilities aren’t mutually exclusive. And I think you can make a case for it going both ways. But even if the left side of the equation is giving orders, that doesn’t necessarily mean Taubes’s insulin-adipose mechanism is the explanation for it.
The second point I’ll make is that it can be difficult to discern which links in the causal chain are giving orders and which links are simply taking orders. In my opinion, this is a major challenge in scientific research. Let’s get back to the car analogy. Imagine that I conduct a study in which I pop the tires of one group of cars, but not a second group of cars, then I measure how fast the two groups drive. Examining the results, I might conclude that tires do in fact control the speed of a car, and therefore that tires are what determine why some cars tend to drive faster than others on my local highway. But even though my experiment demonstrated that tires are in the causal chain, my conclusion would be wrong because I haven’t demonstrated that tires give orders in the context I’m trying to understand.
Similarly, the examples that Taubes and others use to argue that insulin and adipose tissue “give orders” from the left side of the energy balance equation, rather than “taking orders” from the right side, are generally not from the condition of interest, common obesity. They’re from situations in which insulin signaling or adipose tissue function are pushed outside their normal operating parameters by experimental manipulation or disease, much like a car with popped tires: type 1 diabetes, insulin injection, mice with missing insulin genes or genetically manipulated adipose tissue function, etc. These experiments demonstrate that insulin and adipose tissue are in the causal chain of obesity, but not necessarily that they give orders in the context of regular people gaining weight.
When we examine the natural context– people living their normal lives and getting fatter or not– the picture suggests that insulin and adipose tissue are predominantly taking orders rather than giving orders:
- In people living their normal lives, circulating insulin level, and the insulin response to carbohydrate, don’t reliably predict who will gain weight and who won’t. A person with high insulin tends to gain about the same amount of weight over the years as a person with low insulin. It’s hard to understand how elevated insulin could be the primary cause of obesity, given these findings. That said, if insulin were playing a minority role it could be hard to detect in this type of study.
- The most unbiased and informative way I know of determining what’s “giving orders” in a natural context is human genetics research. Genome-wide association studies (GWAS) tell us which locations in the genome are correlated with specific traits in people living their normal lives, and examining the functions of the genes that happen to pop up tells us which systems are “giving orders”. For example, the genes that correlate with type 2 diabetes risk are primarily related to insulin signaling and the pancreas. This tells us that to a large degree, insulin signaling and the pancreas are the biological systems in charge of type 2 diabetes risk. The genes that correlate with height are related to growth of the skeletal system and connective tissues. In obesity, the genes that correlate with body fatness are predominantly related to the brain, not to insulin or adipose tissue. This suggests that in the case of common human obesity, the brain is the primary body system that’s giving orders, and insulin and adipose tissue are primarily taking orders.
This shouldn’t be particularly surprising, since the brain is already known to control both sides of the energy balance equation: it generates eating behavior, including what and how much we eat. It generates physical activity. It regulates metabolic rate. And it’s the only organ in the body known to contain a regulatory system for fat mass.
The brain is the body’s professional information-processing organ and it performs many functions that evolved to satisfy our ancestors’ needs related to energy and food. We can divide these into “homeostatic” and “non-homeostatic” functions that map onto the left and right side of the energy balance equation, respectively. Any control system can fail when placed outside its normal operating parameters, and these systems didn’t evolve for the modern context. Both homeostatic and non-homeostatic brain functions tend to favor overconsumption and maladaptive body fat gain in today’s world, as I describe in my book.
However– importantly– this still doesn’t tell us which side of the energy balance equation is giving orders. It remains possible that the left side is giving orders, but the brain is in charge of it. In fact, this is what people in my corner of the obesity research world (energy homeostasis) have been arguing might be happening for decades: the brain is in control of energy balance, so we should try to understand how that regulatory process is altered in obesity.
How do we answer this question? Let’s consider it from another angle. If energy balance is giving orders rather than taking them, we should be able to bring people from the obese state to the lean state, and from the lean state to the obese state, by changing energy balance alone.
Many studies (and many peoples’ experiences) have shown that reducing calorie intake causes fat loss, and if the cumulative calorie deficit is large enough, people can lose quite a bit of fat. At first glance, this seems like a home run for the argument that energy imbalance gives the orders. Certainly, there’s no doubt that energy imbalance has a powerful impact on fat mass!
However, when we look closer, the situation isn’t quite so cut and dried. The truth is that a person who has lost a substantial amount of fat is not the same as a person who had that leaner body all along. A weight-reduced person has a greater tendency to gain weight/fat, and when we look at this from the perspective of energy balance, we see that they have a hard time maintaining the lower calorie intake, and their metabolic rate has also slowed a bit more than their change in body mass would predict. In extreme cases, such as contestants on the weight loss game show The Biggest Loser, the decline in metabolic rate can actually be quite large. (As an aside, the tendency of a weight-reduced person to regain fat occurs in a state of reduced circulating insulin level.)
This is a phenomenon I call the “starvation response”, and it happens because body fat mass is biologically regulated. Current evidence suggests this regulation happens via the fat-regulating hormone leptin acting predominantly in the brain. Consistent with the genetics data, this is the only known system in the body that regulates fat mass. In my book The Hungry Brain, I review the research on this, which stretches back 180 years but has intensified since the discovery of leptin in 1994.
The “starvation response” to weight loss is an example of the left side of the energy balance equation giving orders, in addition to taking orders. When fat mass declines, there is a reaction from the body that seeks to modify energy balance in order to regain fat.
The situation is similar for fat gain. Increasing energy intake causes fat gain in scientific studies, but it’s hard to maintain substantial gains because the appetite reacts, pushing back against the change in energy balance. Whether or not fat gain via experimental overfeeding also causes a disproportionate increase in metabolic rate is controversial. It’s not difficult to cause a person to gain fat via overfeeding, but it seems to get more difficult the more you want them to gain, presumably because the body fat regulatory system “pushes back”. To some degree, this system protects against both fat loss and fat gain, but it’s obviously less effective at protecting against long-term fat gain in most people.
Insulin does not appear to play a key role in this process because overfeeding carbohydrate vs. fat has about the same effect on fat gain, despite different effects on insulin. Unlike the “starvation response”, the mechanism behind resistance to weight gain remains unclear, and is an active subject of research.
From all this, it’s clear that both sides of the energy balance equation can give orders that affect fat mass, and I think it makes a strong case that both sides do give orders in the context of voluntary weight loss. Yet, is experimental overfeeding an accurate model of the typical fattening process, or are we just popping tires? Does that scenario actually represent the process that causes common obesity in the real world? Here’s the truth: we don’t know for sure.
What we do know is that obesity consists of both a change in energy balance and a change in the biological system that regulates body fatness. Does energy imbalance cause fat gain, and the regulatory system adapts to higher fat mass over time, making it hard to slim back down? Or does the regulatory system go awry in the modern environment, driving fat gain and energy imbalance? Or both? It’s a chicken-and-egg question that we don’t yet have a definitive answer to.
We aren’t flying completely blind though. One way to examine this question is to see whether experimental overfeeding results in weight/fat gain that persists after the overfeeding period, or whether people shed all the excess weight. If people retain excess weight, it would imply that the regulatory system adapts to “defend” the higher level of fat mass, and in turn it would provide evidence that energy imbalance may be giving orders in the development of common obesity.
It turns out that people often do retain part of the excess weight/fat they gain during overfeeding. For example, Johannsen and colleagues overfed 35 young adults for 8 weeks, resulting in 17 pounds (7.5 kg) of weight gain and 9 pounds (4.2 kg) of fat gain. The researchers sent the volunteers home to live their normal lives, then measured their body weight and body composition six months later. At that point, they retained 43% of the excess weight they had gained during overfeeding, and a similar proportion of the excess fat.
This suggests that overfeeding had caused their body fat regulatory system to durably accept a higher “setpoint”. In turn, this implies that energy imbalance was probably giving the orders in this scenario. Some, but not all, other overfeeding studies have reported similar findings.
Experimental overfeeding might still seem like an artificial scenario, but consider that if we’re being honest, most of us “overfeed” ourselves periodically to some degree, meaning we eat/drink more than we need to because the food/drink is seductive, readily available, low-satiety, or for a variety of other reasons. Most annual weight gain in the US occurs during the 6-week holiday period, which represents only 12 percent of the year. On average, we lose some of that excess weight as we come out of the holiday period, but we retain some of it, to be built upon the next year. Our weight ratchets up a bit each year as a result of holiday eating, and the dynamics of it resembles a miniature version of experimental overfeeding.
This evidence isn’t definitive but it does suggest that energy imbalance probably gives orders in the development of common obesity, and therefore energy imbalance probably causes obesity under both definitions of the word “cause”. But this doesn’t exclude the possibility that the left side of the equation is also giving orders.
My guess is that both sides of the equation give orders in the real-life development of common obesity, but that the relative proportions depend on the person. But I don’t know for sure, and I doubt anyone does. This remains an important question for the research community. However, whichever side of the energy balance equation is giving orders, the brain appears to be the commander-in-chief of the equation as a whole.
I conclude the following:
- Energy in/out is causal in obesity, but in and of itself, that fact doesn’t necessarily mean it’s giving orders.
- In terms of modifying fat mass, both sides of the energy balance equation are able to give orders, and also take orders.
- In the context of voluntary weight loss, both sides give orders.
- In the context of people developing common obesity in the real world, we don’t have definitive answers about which side of the equation is giving orders. The evidence suggests that energy imbalance probably gives orders, but we don’t know whether the other side of the equation does as well. I suspect it’s probably both sides and the proportion depends on the person, but that’s just a guess.
- Regardless of which side of the energy balance equation is giving orders, the brain appears to be the commander-in-chief of the equation as a whole.
- Insulin and adipose tissue probably take orders, for the most part, although I don’t think current evidence rules out a minority role for them in giving orders.
Bonus: is the energy balance equation useful?
To those who do obesity research, the value of the equation is obvious: it helps narrow down the possible field of explanations for the body composition of a person or lab animal, or changes in body composition. For example:
- In people with obesity, energy intake and expenditure are typically higher than in people without obesity of the same sex and height. This tells us that common obesity is not the result of a reduced metabolic rate– a common but erroneous belief. It tells us that we should instead focus on mechanisms related to higher energy intake.
- The Hadza are a hunter-gatherer population in Tanzania, and they are characteristically lean. Although they have a higher physical activity level than a typical American, their overall energy expenditure is about the same as a typical American, after adjusting for the fact that their bodies are smaller and leaner. This tells us that higher energy expenditure due to exercise is not the reason they’re lean– we should instead focus on mechanisms related to lower energy intake.
These are just two examples off the top of my head; the energy balance equation has many applications in obesity research. If you want to understand body fatness, it’s valuable to understand the energy balance characteristics of your subjects of interest.
Acknowledgments
Many thanks to Kevin Hall and Karl Kaiyala for their helpful comments on a draft of this post.
Footnotes
*This can be estimated using a few simple assumptions. First, the energy density of fat is 86.9 MJ/lb, and the energy density of lean tissue is 16.72 MJ/lb. Then, assume two men of average height (5’9″), one who is lean (BMI 22, 149 lbs, 12% body fat) and one who has mild obesity
(BMI 32, 217 lbs, 30% body fat). This yields total body energy contents of 3,746 MJ and 8,197 MJ. The body of the man with obesity contains 2.2 times more energy than the body of the lean man, and 92% of the excess energy is contained in fat tissue. The number is not 100% because people with obesity tend to have more lean mass than people who are lean.
**The correlation is strong at R2 = 0.77. Calorie intake was measured over 18 days in a metabolic ward in people who were weight-stable. Data are from table 1 of this study. A similar relationship between BMI and energy intake has been reported in many other studies that measured energy intake accurately but indirectly by measuring energy expenditure in weight-stable people. The first law of thermodynamics (conservation of energy) allows us to convert between energy intake and energy expenditure in weight-stable people.
***This passage from the paper illustrates that the authors do not assume energy imbalance is “giving orders” in the context of common obesity. They describe the question as unresolved:
Another obstacle arises from a lack of scientific consensus regarding fundamental aspects of obesity pathogenesis. During caloric restriction, there is little question that reduced neuronal input from adiposity-related hormones activates responses (increased food intake, decreased metabolic rate) that favour recovery of lost weight. Whether the reverse is also true—that increased neuronal input from these hormones protects against weight gain—is hotly debated. Until this issue is resolved, the importance of several key observations, including CNS resistance to leptin and insulin documented in common forms of obesity, will remain uncertain. This is because if adiposity negative-feedback signals do not normally protect against weight gain, neuronal resistance to insulin and leptin cannot cause obesity. According to this view, the growing obesity epidemic can be attributed to an inherent lack of protection against obesity-promoting environmental factors, rather than to an underlying homeostatic disorder. Alternatively, if adiposity negative-feedback signals do, in fact, confer protection against obesity in normal-weight individuals, neuronal resistance to these signals must, by definition, favour weight gain, and unravelling the underlying causes takes on both pathophysiological and therapeutic urgency. As our understanding of normal and abnormal regulation of food intake and body adiposity grows in its sophistication, overcoming these obstacles will create new opportunities for therapeutic intervention.
Morton et al. Nature 443:289. 2006


I’ve followed this debate (and both you and Gary), for over a decade. It’s certainly lead to fruitful and interesting debate.
Regarding your point above body mass and energy intake; wouldn’t we expect fat people to eat more due to the fact they are having to carry more weight around? (Apologies if this is a dim question).
Sort of. The main thing that determines energy requirements is lean mass. Fat tissue itself has very low energy requirements. People with obesity tend to have more lean mass and so they eat more energy and expend more energy. People gain lean and fat mass when they gain weight, and when they lose weight they also lose both.
Stephan, this is a very interesting review. I wonder if you would address the following topic –
Suppose we have a (non-malfunctioning) person whose systems “want” to maintain a particular weight. How can that person know how much energy is needed to make up for the day’s expenditures. I’m thinking that it would be difficult for the body/brain to measure both the energy expenditure and the amount of energy that will be acquired by digestion of the day’s food with enough accuracy to keep them in balance. Since an imbalance of only 100 Calories per day – that’s roughly 5% – would result in around 10 pounds (4.5 kg) weight change in a year, it’s important to understand this.
The regulatory system has at least two levels. There’s a short-term regulatory system that determines food intake on a meal-to-meal level and a long-term system that sets the gain on energy balance based on fat mass. I think the short-term system gets you in the right ballpark and then the long-term system tweaks things over longer periods to keep fat mass relatively stable.
I am assuming the subjects in the plot of BMI were weight-stable during the time of the measurement. If so, the plot has nothing to do with the energy equation presented. The equation presented says 0 = Energy in – Energy out, or rearranging terms, Energy in = Energy out.
So people whose BMI is higher both consume and use more energy. OK. But we are wondering why they are heavier. Presumably at some point in the past they had a positive energy balance. But if the measurements were taken at a time that they were weight stable, those measurements don’t really shed light on what happened in that earlier time.
I know this is a tiny part of your excellent post – which I am still digesting. I just wonder if the post would be stronger without the plot?
Regarding the energy balance equation itself, I don’t think it is particularly useful in most contexts. It can be, frankly, a distraction. I think your focus on how the brain is regulating the body, and what external stimuli (say, bland food) may cause the brain to adjust its set point, is far more fundamental to what is going on.
In your thermostat analogy, there is a set point. If the room is too cold, the thermostat sends a signal to the heating system to add heat. This is the interesting part. The exact details of the heating system’s output in BTU/hr into the room relative to the energy loss through conduction through the walls & etc. is not that useful for understanding the system at a functional level, in most instances.
These exact details of energy into the room less the energy out of the room, taking into account the heat capacity of the entire system, drive the resulting temperature change (in other words, a perfect analogy to your energy equation). At times, an engineer or a plumber will want to know those things, at least roughly, to size the heating system and specify the insulation and etc. But to generally understand how the control system works, we don’t need to put too much focus on that.
There are also details about how the signal from the thermostat is transferred to the heating system. Older systems use 2 wires, and I could provide additional detail, but unless we were troubleshooting a malfunctioning system, you probably don’t care. Of course if someone cuts these wires you have got to fix them or your house will freeze.
This may be an analogy to insulin – assuming an otherwise healthy person, it is similar to the wires between the thermostat and the furnace. We may not care much about those details when trying to understand the control system. But, of course, if there is a problem with insulin (example: Type 1 diabetic) we better fix it right away.
Thank you for your interesting post. I really enjoyed your book.
Thanks for your comments. Yes, they were weight stable. The graph serves to illustrate two major points. 1) We don’t have to make assumptions about energy intake, as implied by Gary’s statement “How do we know we’re overeating? Because we’re getting fatter.” These things can be measured. Gary often writes as if obesity researchers blunder through causal inference using obtuse logic rather than experiments. 2) If we know that energy intake is positively correlated with BMI, and we know that reducing energy intake back to the lean level causes substantial fat loss, that puts the spotlight on elevated energy intake rather than reduced energy expenditure as the key causal factor. And again that highlights the value of measuring things rather than making assumption.
I’m not sure how much practical value the energy balance equation has for the average person, but I do think it’s at the heart of some scientific questions, which is why Gary spends so much time on it. From his perspective, if energy intake isn’t elevated in obesity (as he has incorrectly argued), then obesity can’t be caused by excess energy intake, and the focus changes from energy intake to whatever metabolic state is causing a person to have a slow metabolism (per unit tissue mass). This idea would be easier to reconcile with his insulin hypothesis.
He spends many pages in GCBC trying to convince the reader that obesity in animals and humans is not generally associated with higher energy intake, and that in fact energy intake is not even a very relevant variable for body fatness. The studies he cites on that are either not rigorous (humans) or misrepresented (rodents). I reviewed the historical rodent lit for my book and it’s clear that body fatness is strongly (but not perfectly) related to energy intake in the experimental models he discusses.
>From his perspective, if energy intake isn’t elevated in obesity (as he has incorrectly argued), then obesity can’t be caused by excess energy intake, and the focus changes from energy intake to whatever metabolic state is causing a person to have a slow metabolism (per unit tissue mass). This idea would be easier to reconcile with his insulin hypothesis.
I’m not so sure it would, given that in the situations where metabolism is indeed slowed (weight loss by direct calorie restriction, as opposed to ad libitum), insulin is also low. I’d actually expect low insulin (high sensitivity) to predispose one to weight gain, ceteris paribus.
Don’t get tired, Stephan. It happens to Taubes like some of the ancient philosophers, who tried to understand and explain reality without knowing science. The result was either mental gibberish or nonsensical theories. Or both, as is the case.
The Energy-Out Side of the Energy Balance Equation is Fixed and Unchangeable (long-term)
Total Energy Expenditure (TEE) in Humans is Fixed
Thesis:
Total Energy Expenditure (TEE) in humans, long-term, is strictly regulated to keep body temperature at 98.6 degrees – no more and no less. This implies that the “total calories burned” part of Energy Balance cannot be changed by ordinary exercise – or any clever physiological ruse.
This preliminary paper sketches out the physiological mechanisms controlling the empirical results.
Background:
Almost universally, expert opinion advocates losing weight through controlling energy balance by diet and exercise. However, a smaller group points out that in trial after trial exercise has proven ineffective (most recently and convincingly by Pontzer et al). However, even exercise skeptics have relied upon trial results, rather than causal physiology.
Additionally, researchers and authors have put forth claims that non-exercise methods can increase metabolic rates, thus burning more total calories, to prevent and treat obesity and the Metabolic Syndrome. In fact, substantial research funds have been dedicated to these objectives.
Explanation:
Dominant physiological mechanisms exert long-term control over Total Energy Expenditure (TEE) – total calories burned per day – and make it impossible to substantially alter the total number of calories burned. Yes, this unconscionably means that the regular exerciser and the slothful couch potato will burn nearly the same number of calories.
Conventionally, TEE is estimated by adding up all the various calorie burning functions: brain, muscle ATP production, body maintenance, bodily functions, food processing and physical work. For example, it is supposed that female muscles burn fewer calories than male muscles because they are smaller, erroneously explaining lower female TEE.
Then it is believed that increasing any of these, especially physical work, will increase TEE. But, in fact, although short-term TEE may increase (additive), long-term TEE will not increase (constrained) – because a change in magnitude of any component of TEE will, long-term, cause a compensatory change via specialized calorie burning mechanisms which add just enough heat to maintain body temperature at 98.6 degrees (37 degrees C).
Each calorie burned for “base” metabolic purposes produces almost a calorie of heat. (Calorie burning for structural growth or maintenance may differ.) This heat is not a meaningless byproduct which disappears into the ether, it becomes part of total body heat which equates to body temperature.
So then, body temperature must stay anchored to 98.6 degrees and any variations of “base metabolism” (generates ATP and heat) must be automatically counterbalanced by regulatory mechanisms which add heat as required – “complementary metabolism” (generates no ATP, just heat).
Medical physiology texts say that body temperature is regulated by perspiring if too warm and shivering if too cold. Unfortunately, the most cursory observation of humans shows them to be neither perspiring nor shivering. Fortunately, researchers in the body temperature universe have tentatively identified mechanisms which do regulate body temperature.
Temperature detection and the 98.6 set point, once thought to be entirely located in the hypothalamus, are now considered to be the product of hypothalamic integration of signals from a substantial number of diversely located, diversely responsive nerve cells. Of course, this same hypothalamus controls the level of thyroid hormone.
As mentioned, the mechanisms affecting TEE are both short-term, which seem additive; and long-term, which are constrained. This long-term, short-term dichotomy reflects the slow response time (weeks – typical of “adaptive” responses, like changes in gene expression) of the thyroid based negative feedback control system dedicated to generating just enough additional heat to maintain body temperature at 98.6.
While the exact physiology of the “complementary” heat generation and its control system are unclear, even controversial, its actions via uncoupled heat generation are acknowledged. Uncoupled heat generation in mitochondria is quite purposeful, it does not generate ATP, just enough additional heat to maintain 98.6.
Two sites of complementary heat generation are proposed. Mitochondrial uncoupling in Brown Adipose Tissue (BAT) is the source of complementary heat in mice, regulated by Uncoupling Protein 1 (UCP1) via creation of proton-motive force. Adult humans were thought to lack brown fat, but recent discoveries of some deposits have steered researchers in that direction – though it’s possible that they have spent too much time in the company of mice.
Heat generation in BAT occurs via SNS activity, with norepinephrine triggering cellular pathways that cause mitochondrial heat production. But it is important to understand the role of thyroid hormone since the slow time constant of its homeostatic feedback system causes deceptive delays in observing the effects of adaptive complementary thermogenesis. In mice, thyroid hormone increases thermogenesis in BAT via increased responsiveness to norepinephrine, as well as enhancing the cAMP-mediated rise in UCP1 gene expression.
In humans, the alternative site, which some researchers consider discredited, is skeletal muscle mitochondria, regulated by Uncoupling Protein 3 (UCP3). Thyroid hormone regulates expression of UCP3 while body temperature regulates thyroid hormone – a closely regulated, but slow-acting, feedback control system.
It is worth noting that total body heat differs from total heat generated since, like your hot water heater, depending on the quality of insulation, heat is lost. If you check your catalog, you will discover that human hot water heaters come in two models – male and female. Females are delightfully better insulated via subcutaneous fat. This diminished heat loss allows females to maintain 98.6 at a lower TEE – although other factors, like body mass, also contribute.
Extraordinary cooling from perspiration of lightly clothed athletes or from a coolant, like Michael Phelps’ swimming pool, can add to conventional heat loss. Of course, neither of these conditions is significant in ordinary exercise.
Tales of teenagers or bodybuilders consuming high-calorie diets without adding body fat indicate that growth or repair generates less heat for each calorie used than does production of ATP.
Doubt:
While it is understandable that obesity researchers might not connect body temperature with proposed manipulation of metabolic processes, like exercise; it seems quite remarkable that peer-reviewed articles dedicated to explicating the mechanisms controlling body temperature should, time after time, also propose that therapeutic increases in brown fat metabolism might tip the energy balance equation in favor of reduced obesity.
They would be wise to note that in the 1930s this was attempted using a mitochondrial uncoupler to override our constrained heat generation. Unfortunately, and predictably, there were fatalities caused by rapid overheating.
Because the nomenclature in the literature seems inconsistent (base, obligatory, facultative), I have used “base” to describe metabolism aimed at ATP generation and the internally descriptive “complementary” to denote metabolism whose function is limited to adding enough heat to top off accumulated body heat at 98.6-degree levels.
————————————————–
References
Guyton and Hall Textbook of Medical Physiology 13Ed 2016
In discussing many of the metabolic reactions in the preceding chapters, we noted that not all the energy in foods is transferred to ATP; instead, a large portion of this energy becomes heat. On average, 35 percent of the energy in foods becomes heat during ATP formation. Additional energy becomes heat as it is transferred from ATP to the functional systems of the cells, so even under optimal conditions, no more than 27 percent of all the energy from food is finally used by the functional systems.
Even when 27 percent of the energy reaches the functional systems of the cells, most of this energy eventually becomes heat. For example, when proteins are synthesized, large portions of ATP are used to form the peptide linkages, and this action stores energy in these linkages. However, continuous turnover of proteins also occurs—some are being degraded while others are being formed. When proteins are degraded, the energy stored in the peptide linkages is released in the form of heat into the body.
Another example is the energy used for muscle activity. Much of this energy simply overcomes the viscosity of the muscles or of the tissues so that the limbs can move. This viscous movement causes friction within the tissues, which generates heat.
Consider also the energy expended by the heart in pumping blood. The blood distends the arterial system, and this distention represents a reservoir of potential energy. As the blood flows through the peripheral vessels, the friction of the different layers of blood flowing over one another and the friction of the blood against the walls of the vessels turn all this energy into heat.
Essentially all the energy expended by the body is eventually converted into heat. The only significant exception occurs when the muscles are used to perform some form of work outside the body. For instance, when the muscles elevate an object to a height or propel the body up steps, a type of potential energy is created by raising a mass against gravity. However, when external expenditure of energy is not taking place, all the energy released by the metabolic processes eventually becomes body heat.
Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans
Pontzer, Herman et al.
Current Biology , Volume 26 , Issue 3 , 410 – 417
http://www.cell.com/current-biology/fulltext/S0960-9822%2815%2901577-8
Thyroid Hormone Regulation of Metabolism
Rashmi Mullur, Yan-Yun Liu, Gregory A. Brent
Physiological Reviews Published 1 April 2014 Vol. 94 no. 2, 355-382 DOI: 10.1152/physrev.00030.2013
http://physrev.physiology.org/content/94/2/355
Thyroid Hormone and Central Control of Metabolic Homeostasis
Warner, A. and Mittag, J. (2012). Thyroid hormone and the central control of homeostasis. J. Mol. Endocrinol. 49, R29-R35.
Review Article
Ines Donangelo
http://www.jscimedcentral.com/Endocrinology/endocrinology-spid-role-thyroid-hormone-metabolic-homeostasis-1047.pdf
Muscular efficiency during steady-rate exercise: effects of speed and work rate
G.A. Gaesser, G.A. Brooks
Journal of Applied Physiology, 38 (6) (1975), pp. 1132–1139
http://jap.physiology.org/content/38/6/1132
The linchpin of Energy Balance is upended – and nobody cares.
Interesting.
Thank you Stephan,
You write “That’s how we know increasing energy intake increases body fatness, regardless of whether the excess energy comes from carbohydrate or fat.” I do not see the point here. What does this have to do with something being the cause of anything? While we know it is possible to voluntarily increase energy intake for a short time period and gain weight, I cannot see it has anyhting to do with the question: Is the cause for the increase in obesity the last decades partly caused by people eating too much? For all practical purposes, it doesn’t add anything to what the mechanisms behind the increase in obesity are, and what we should do about it, as I see it.
Where is the data showning that somebody normal weight who eats the same type of healthy food (what ever that is) for an extended period and tries to eat 500 kcal extra per day or so ends up being obese? Maybe an impossible experiment, but I cannot see that you your post have provided any arguments that would back up overeating being a cause of obesity.
Hello Inge, I address your first question in the post.
As for your second question, I don’t know what you consider to be “healthy food”. It’s possible that overconsuming some types of food causes more retention of excess weight than others, but that hasn’t been demonstrated in humans. In rodents, there is some evidence that diet quality, in addition to quantity, influences obesity development. The rodent data suggest a two-hit mechanism that requires both hyperleptinemia (as would occur with overeating) and diet quality. This suggests that overconsumption per se is necessary, but not sufficient, for obesity development. But this needs to be tested in humans.
Hi, heat plays a role as indicated above, what can we learn from heavy drinkers: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1530-0277.1997.tb04238.x
Addolorato on energy expenditure with chronic alcoholism 1997
Slim italians alcoholics vs. moderate users were compared:
50-70kg bmi less than 25 for both
BMR kcal / kg 27.1 vs 23.4 i.e. +16% for alcoholics
lipid oxidation 550 kcal/d vs. 250 kcal/d i.e. +200%
intake kcal/d 3140 vs 2530 i.e. + 24% for alcoholics (more; they were 10% lighter by means)
ethano kcal/d 1330 vs 273 i.e. 2litres of wine, >40% of E for alcoholics
Heavy alcoholics are not fat, more wasted by clinical experience. They suspect that microsomal pathway becomes dominant for alcohol metabolism and creates HEAT plus fat deposit shift, in addition to ketones and cardiac output increase. (note; short term overuse does not do this, they suspect mitocondrial adaptation). Feeling warm while getting high on alcohol, anybody? This group should measure body temperature next time, maybe.
In a way this resembles latest Hall study, where LC arm ate more but also TEE was more.
Since no one eats exact calories days in, but many remain weight stable, what is the regulation (besides heat production)?
JR
Does the recent years gut microbiome research come into play regarding the microbiome perhaps affecting the brain via the vagus nerve or other mechanisms?
Energy in = energy out ?
How is energy_out defined?
In context of obesity it might be better to rewrite the above equation as
Energy in = energy out + energy stored
Obesity is then a disorder in which body stores more energy than it should. The diversion of the energy_in into stored energy is presumably under hormonal control and thus obesity is a hormonal disorder. As insulin promotes energy storage, one might assume insulin to be involved in the disorder.
Obesity is a hormonal disorder in the sense that the leptin-dependent energy homeostasis system is altered. You are implying that obesity results from a hormonally-controlled alteration of energy partitioning, but the evidence doesn’t support that. Energy expenditure per unit lean mass is normal in people with obesity.