This is a list of references I am likely to cite in my debate with Gary Taubes on the Joe Rogan Experience on March 19. During the debate, I’ll be calling out numbers corresponding to the references below. Scroll down to the number I mentioned to see the references for yourself.
Gary and I prepared a document explaining our models that you can download here. For context, I am favorable toward low-carbohydrate diets, but the references below mostly reflect issues that Gary and I are likely to disagree on.
The brain
1. The brain regulates body fatness
This is supported by a mountain of evidence going back 180 years. The brain, and particularly a non-conscious part of the brain called the hypothalamus, contains the only known system in the body that regulates body fatness. Damage to specific parts of the hypothalamus cause severe obesity or leanness in animals and humans, and experimentally controlling the activity of specific groups of neurons alters body fatness in animal models. See these review papers for an overview of some of what we know. I also give a lay-friendly overview of this research in my book The Hungry Brain.
Nearly all anti-obesity drugs that are currently approved by the FDA target the brain, including liraglutide, lorcaserin, phentermine/topiramate, and naltrexone/bupropion. The only exception is orlistat, which reduces dietary fat absorption in the gut. Interestingly, one of the most effective of these weight loss drugs, liraglutide, increases post-meal insulin secretion.
2. The genetics of obesity point to the brain, not fat cells or insulin, as the primary determinant of body fatness
Obesity risk is strongly influenced by genetics, and recent studies have begun to uncover the specific genetic differences that make some people fatter than others. This gives us the opportunity to see what these genes do, providing a window into the causes of common obesity. Results show that gene variants that impact body fatness are primarily related to brain function— not primarily insulin or fat cells (although many mechanisms may contribute to a smaller degree). Importantly, these (GWA) studies are unbiased because they examine the whole genome without preconceived notions about which genes or biological pathways might be important.
The largest and most recent study on the genetics of body fatness concluded that “[body mass index]-associated genes are mostly enriched among genes involved in neurogenesis and more generally involved in the development of the central nervous system.”
3. The brain regulates appetite
This should be obvious, because appetite is a motivational state that is generated by the brain– like all motivational states. In fact, we know so much about how appetite-regulating brain circuits work that researchers can turn appetite on and off at will by controlling specific types of neurons in animals. Here are a few review papers that give a broad overview of much of what we know. I give a lay-friendly overview of this research in my book The Hungry Brain.
4. The determinants of appetite are complex and cannot be reduced to glucose and insulin
The brain determines hunger and satiety by in response to signals it receives from inside and outside the body. From inside the body, the brain receives information about the body’s energy status. This comes from neural signals and hormones ascending from the small intestine, pancreas, liver, and fat tissue that tell the brain how much food is in the gut, what type of food it is, and how much fat the body contains.
From outside the body, the brain receives signals about what food cues surround you, and this can also stimulate hunger (think about smelling pizza). All of this information is integrated by the brain, which uses it to generate the overall feeling of hunger or satiety. For a review of some of the research on this see this paper.
5. Lean people, and people with obesity, both “defend” their current level of body fatness against fat loss
When people lose fat, their brains initiate a sort of “starvation response” that favors the regain of lost fat over time. This is familiar to most people who have dieted: increased hunger, more cravings, reduced metabolic rate, and eventual weight regain. This is the brain’s response to fat loss, regardless of whether a person starts off lean or with obesity. This suggests that the “setpoint” around which body fatness is regulated is increased in obesity, since the brain nonconsciously “defends” the obese state. This is one of the key reasons why weight loss is challenging and often temporary.
We know this effect is due to a decline in the fat-regulating hormone leptin during weight loss, because replacing leptin to the pre-weight-loss level largely prevents this “starvation response”. Therefore, the reason people with obesity “defend” a higher level of fat mass is that their brains require a higher level of leptin to feel like they aren’t starving.
6. Single-gene mutations that lead to obesity in humans act in the brain
A number of single-gene mutations have been identified that lead to severe obesity in humans and animals. These are all related to the brain systems that regulate body fatness, and particularly the fat-regulating hormone leptin. I’m not aware of any mutations in insulin or fat cell-related genes that cause obesity.
Calories
7. People with obesity eat, and expend, more calories than lean people
Studies using accurate methods consistently show that people with obesity eat, and expend, about 20-35 percent more calories than lean people, after correcting for height, sex, and physical activity level. Calorie intake and expenditure increase continuously in parallel with body weight. Since calorie intake is equal to calorie expenditure in a weight-stable person, measuring calorie expenditure is the simplest and most accurate way to demonstrate that intake and expenditure are both elevated.
8. Reducing the calorie intake of a person with obesity to that of a lean person causes weight loss
People with obesity consume about 20-35 percent more calories than lean people. Tightly controlled studies have demonstrated that restricting calorie intake by about this much in people with obesity causes rapid weight and fat loss, demonstrating that the elevated calorie intake is required to sustain the obese state.
9. Calorie intake, not carbohydrate intake, is the main dietary determinant of body fat loss
Several controlled feeding studies have compared the effect of calorie-restricted diets with different proportions of fat and carbohydrate on weight and fat loss. Fat loss is determined primarily by the number of calories in the diets, not the proportion of fat or carbohydrate, even when insulin levels differ substantially. This was confirmed by a recent meta-analysis (study of studies) of 20 studies, which concluded that “for all practical purposes ‘a calorie is a calorie’ when it comes to body fat”.
10. Changes in body fatness as a result of diet depend primarily on calorie intake, not carbohydrate intake
This was demonstrated by a meta-analysis (study of studies) of 20 tightly controlled studies in which people were fed diets that differed in carbohydrate and fat content, but not in protein or calorie content. In other words, same calories, different carb:fat ratio. Calorie-for-calorie, higher-carbohydrate diets led to slightly more body fat loss than higher-fat diets, but the difference was very small. The researchers concluded that “for all practical purposes ‘a calorie is a calorie’ when it comes to body fat.”
11. Energy expenditure (metabolic rate) is scarcely affected by differences in carbohydrate and fat intake
This was demonstrated by a meta-analysis (study of studies) of 28 tightly controlled feeding studies. Altering the proportion of carbohydrate to fat in the diet had very little impact on metabolic rate when calorie and protein intake were the same, although metabolic rate was slightly higher on higher-carbohydrate diets.
A recent study received a lot of attention for finding that a very-low-carbohydrate diet led to a higher metabolic rate (+209 kcal/day) than a low-fat diet in people maintaining weight loss. While this study was rigorous and innovative in many ways, some of the data it collected are clearly erroneous because they appear to break the first law of thermodynamics, which is physically impossible. Once the erroneous data are removed, the effect size shrinks, loses its statistical significance, and becomes similar to the other 28 studies that were conducted before it.
Carbohydrate, sugar, and fat
12. Low-fat, high-carbohydrate diets cause weight loss, even without deliberate portion control
The most rigorous study on this is the DIETFITS randomized controlled trial, which was partially funded by Gary Taubes’s organization NuSI. In 609 people, a whole-food low-fat diet resulted in 12 pounds (5.3 kg) of weight loss at one year, not significantly different from the 13 pounds (6.0 kg) of weight lost on a whole-food low-carbohydrate diet. Neither diet included advice to restrict calorie intake. The low-fat diet supplied about twice as much carbohydrate, and 1.5 times as much sugar, as the low-carbohydrate diet, although neither diet was high in sugar. It also had about twice the glycemic load.
Another study conducted at the University of Washington tested the effect of dietary fat restriction on calorie intake and body weight in 16 overweight people using very careful measurements. When they were transitioned to a low-fat diet (15%) and told to eat as much as they wanted, their daily calorie intake dropped by 291 calories and they steadily lost weight and body fat over a 12-week period, losing 8 lbs (3.8 kg) total. Importantly, their total carbohydrate intake increased from 253 grams per day to 318 grams per day. Here’s the graph showing daily calorie intake and weight change (circles = calorie intake, diamonds = weight):
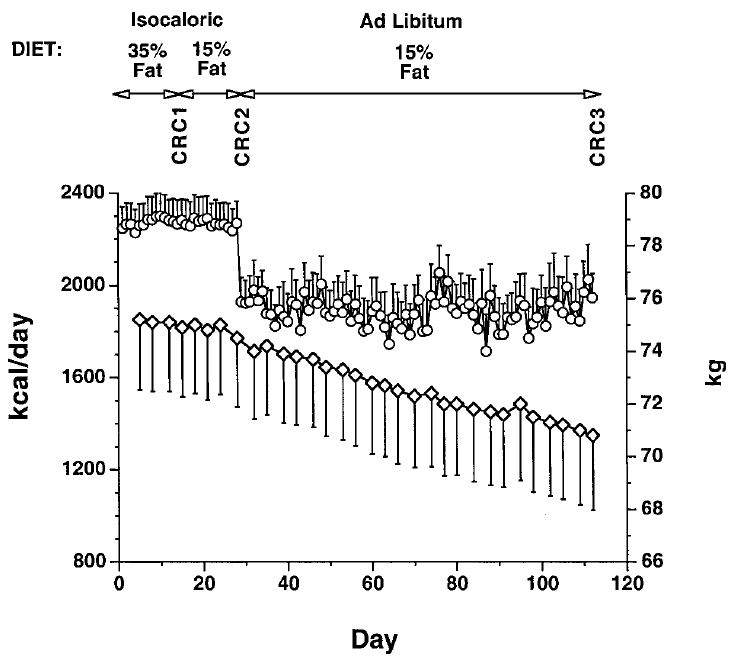
A meta-analysis (study of studies) representing 16 low-fat diet studies also reported that low-fat diets without deliberate portion control cause weight loss.
13. A low-calorie diet composed almost entirely of refined carbohydrate, including sugar, causes substantial weight loss
This paper discusses 106 patients who lost an average of 140 pounds (64 kg) and improved their blood sugar control eating the “rice diet”, a diet composed almost entirely of white rice, fruit, juice, and white sugar. Here is a photo of one man before and after the diet, from the paper:
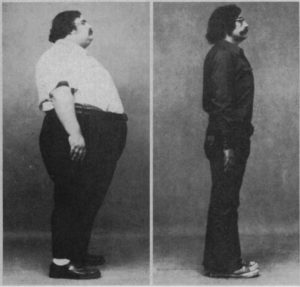
The weight loss version of the rice diet was low in calories, supplied over 200 grams of mostly refined carbohydrate per day, and patients also increased physical activity. This demonstrates that refined carbohydrate does not override the impact of calorie intake and physical activity on body fatness.
The rice diet was also effective for treating diabetes, whether or not it was calorie-restricted.
14. Dietary fat can be fattening in a variety of nonhuman species
A review paper on this topic states that “With few exceptions, obesity is induced by high-fat diets in monkeys, dogs, pigs, hamsters, squirrels, rats, and mice.” There are hundreds of studies demonstrating this, but the most conclusive one was published in 2018 by John Speakman’s lab. They fed five different strains of mice diets varying in fat, carbohydrate, and protein content and showed that body fatness was primarily linked to the amount of fat the mice were eating. Body fatness increased as dietary fat increased up to 60% of calories, and then body fatness declined as dietary fat increased further (this is consistent with other research showing that the very-high-fat, very-low-carb ketogenic diet promotes leanness in mice, as it does in humans). Diets high in sugar and refined starch were not particularly fattening. The takeaway is that the most fattening diet contains abundant fat and carbohydrate together, while diets at the extremes (very-low-fat and very-low-carb) are more slimming.
15. Dietary fat can be fattening in humans
This really depends on context, since low-carbohydrate, high-fat diets tend to be slimming relative to diets that are high in both carbohydrate and fat. Nevertheless, unrestricted diets rich in fat tend to lead to higher calorie intake and fat gain relative to unrestricted diets rich in carbohydrate. This seems to be related to the high calorie density and palatability of fat.
Overfeeding fat under controlled conditions also causes body fat gain.
16. Fat and carbohydrate are equally fattening when overconsumed
Two randomized controlled trials have reported the effect of overfeeding the same number of calories of high-carbohydrate vs. high-fat diets in humans, and both reported equal fat gain.
- Horton and colleagues increased the calorie intake of volunteers by 50 percent by adding only fat vs. only carbohydrate for 14 days under tightly controlled conditions, and measured changes in body fat mass using gold-standard hydrostatic weighing. After 14 days, both groups had gained 3.3 lbs of fat, despite higher insulin levels in the high-carbohydrate group. In the high-carbohydrate arm, most of the extra carbohydrate was refined starch and sugar. In the high-fat arm, most of the extra fat was dairy fat (cream, butter).
- Lammert and colleagues increased the calorie intake of volunteers by 1,194 calories per day for 21 days under tightly controlled conditions. Subjects were fed either a high-carbohydrate (78% CHO, 11% fat) or a high-fat (31% CHO, 58% fat) diet containing the same amount of calories and protein, and the researchers measured changes in body fat mass using gold-standard hydrostatic weighing. After 21 days, both groups had gained 1.8-1.9 lbs of fat.
17. Sugar intake has been declining for 20 years in the US and 50 years in the UK, while obesity and diabetes rates have risen
Here is a graph showing sugar intake and the obesity rate in the US between 1980 and 2013. The sugar data include all added sugars such as table sugar, honey, and high-fructose corn syrup, but not sugars naturally occurring in fruits and vegetables.
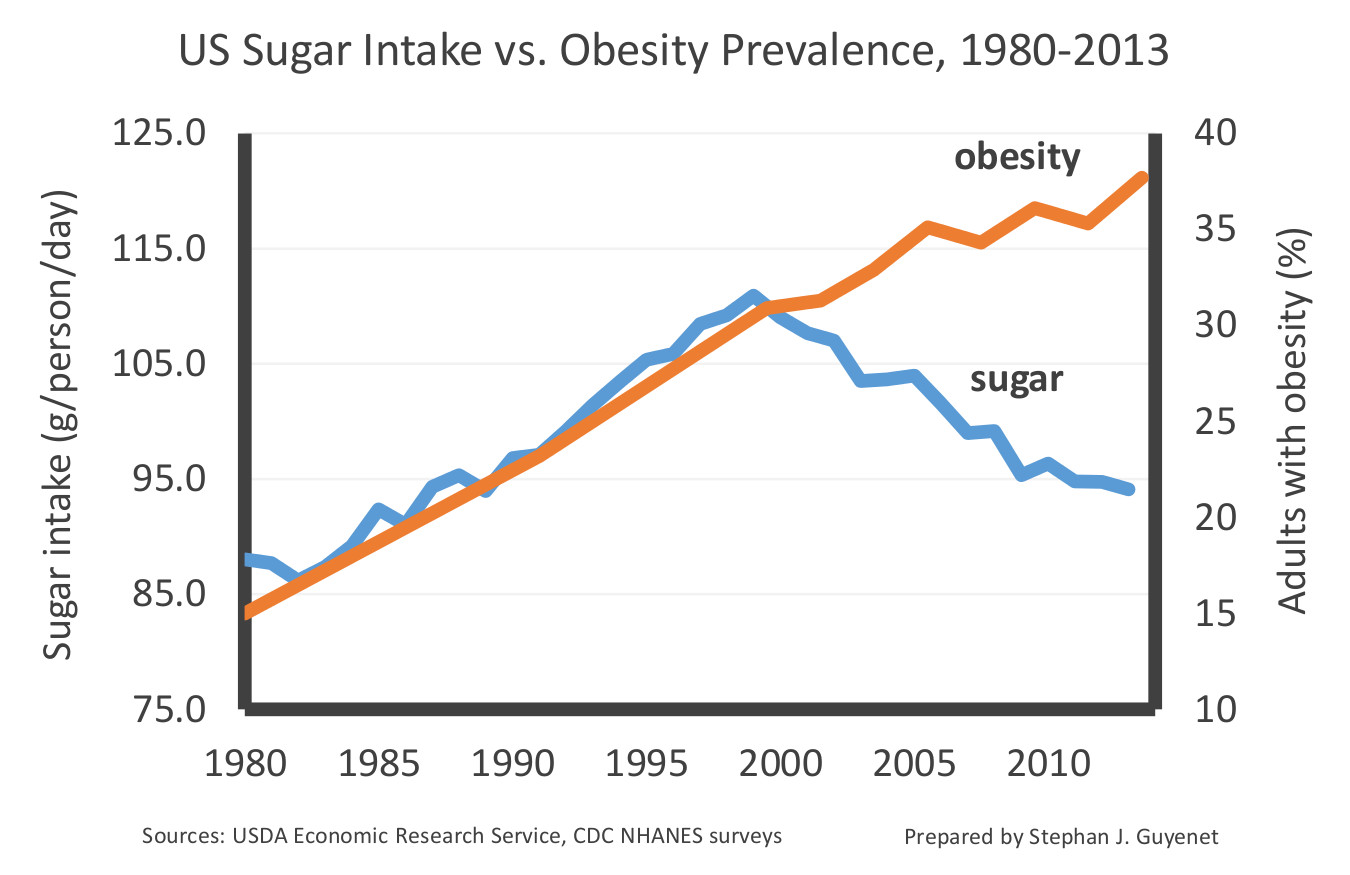
This graph illustrates the lack of correlation between sugar intake and obesity rates after 1999. This decline in sugar intake has been confirmed by several independent lines of evidence. The data suggest that sugar intake has dropped by 15-23 percent over the last two decades, primarily because people are drinking fewer sweetened beverages. Diabetes rates have also increased during the decline in sugar intake.
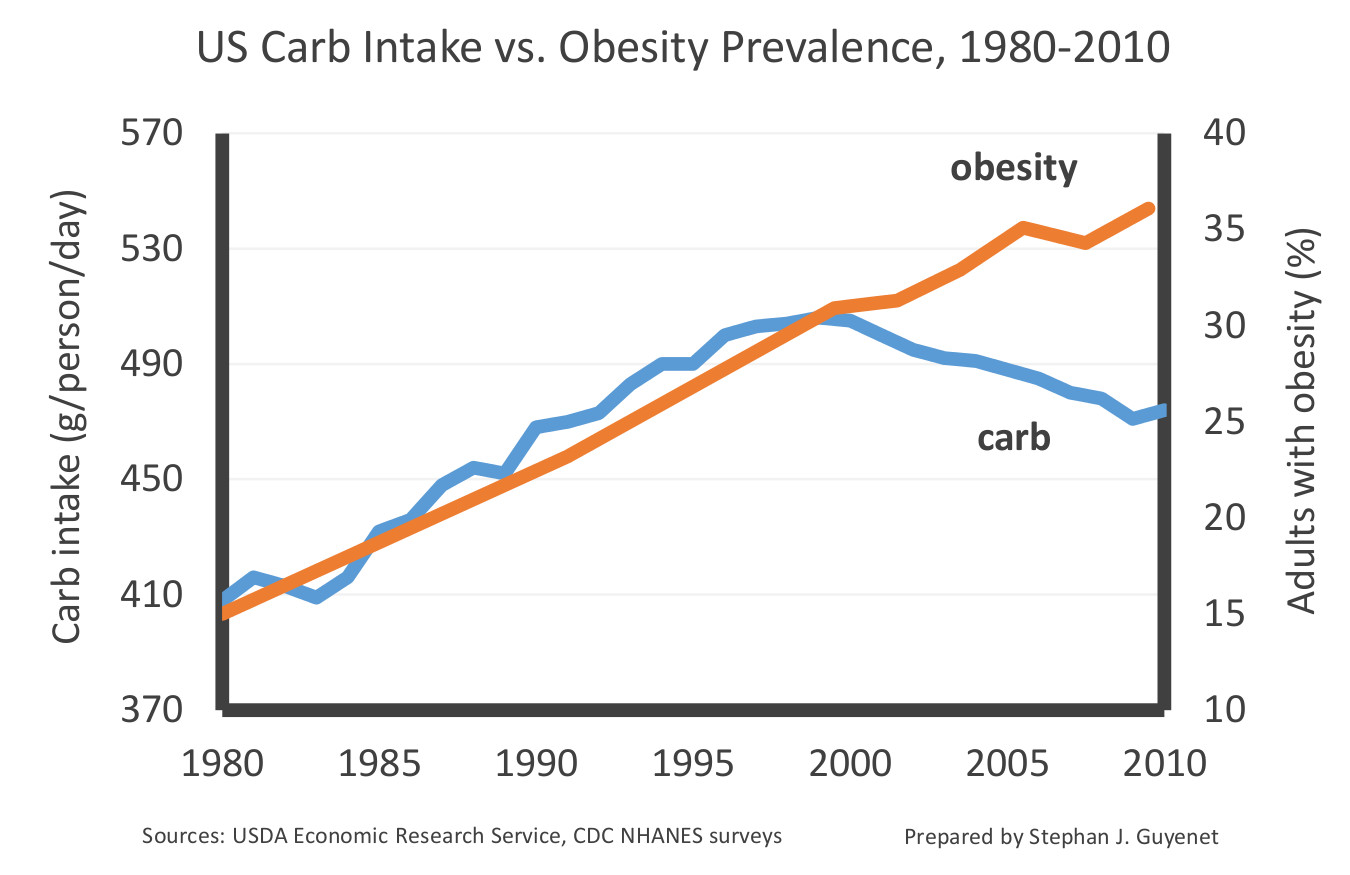
Overall carbohydrate intake has also declined over the last two decades in the US:
This graph shows that sugar intake has declined by 22 percent in the UK over the last 50 years, while obesity and diabetes rates have increased substantially (prepared by Kevin Bass):
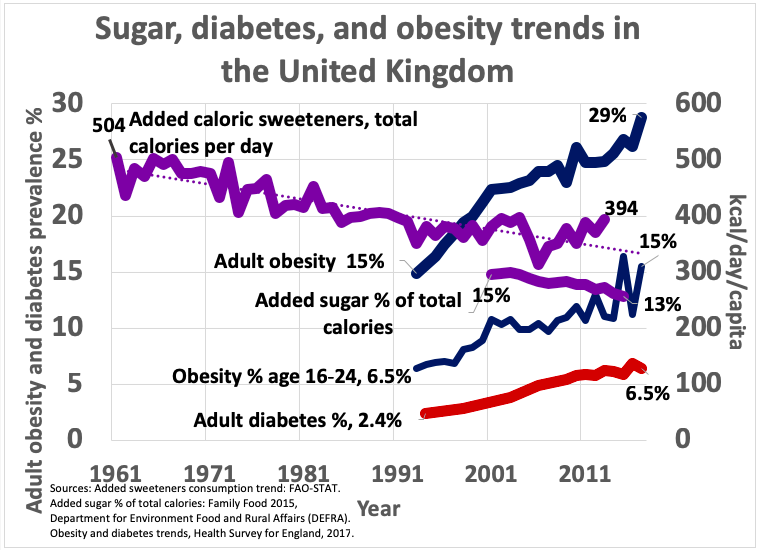
18. Carbohydrate, fat, and protein intake in the US over the last century
This graph represents absolute carbohydrate, fat, and protein intake in the US since 1909. It’s based on US Department of Agriculture data.
Note that fat is the only macronutrient that has consistently and substantially increased over time. Carbohydrate intake declined in the 1940s-50s, rose again in the 1980s-90s, and has been declining since 1999.
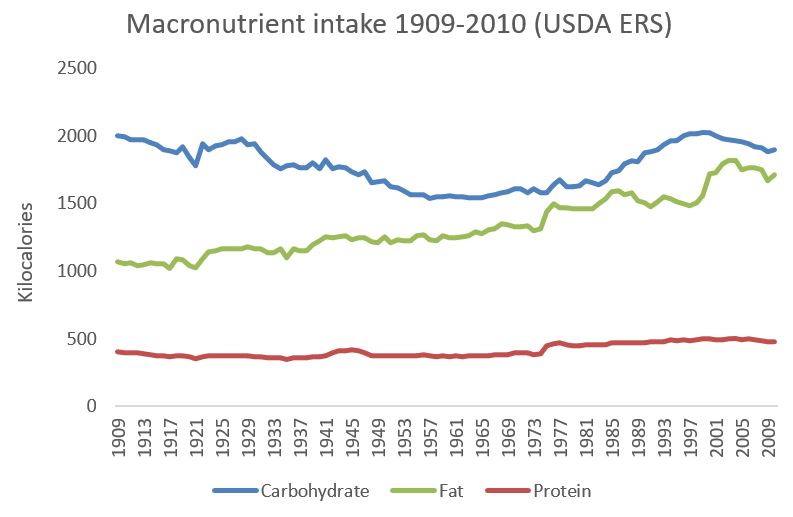
Here are the same data, represented as percentages of total calorie intake:
Aside from a little bump in the 1980s and 1990s, as a percentage of calories the US diet has progressively become higher in fat and lower in carbohydrate.
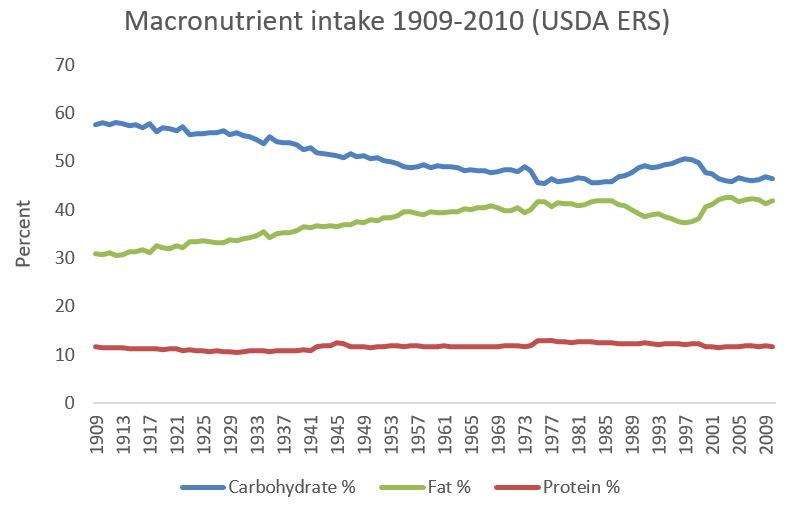
19. White flour intake was much higher in 1900 in the US than it is today
I downloaded this graph directly from the US Department of Agriculture website, although the web page I downloaded it from is no longer online. It speaks for itself. The large majority of this flour would have been refined, white flour from the late 1800s onward.
20. Sugar intake in the US, 1822-2016
This graph represents added sugar intake, including honey and high-fructose corn syrup but not fruit sugars, from several data sources:
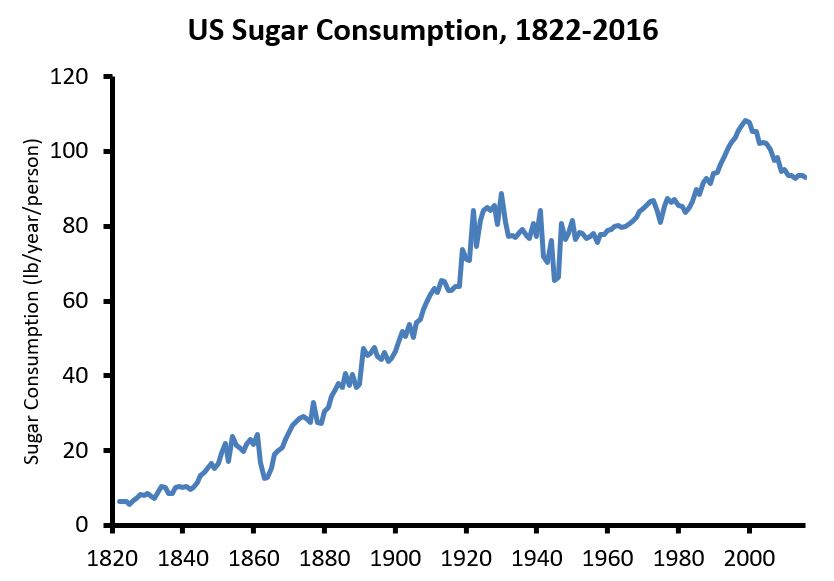
21. Cultures with high intakes of sugar but otherwise healthy diets and lifestyles do not have obesity or diabetes
A well-studied Tanzanian hunter-gatherer population called the Hadza gets 15 percent of its average year-round calorie intake from honey, plus fruit sugar on top of it. This approximates US sugar intake, yet the Hadza do not exhibit obesity, diabetes, or cardiovascular disease.
Another mostly hunter-gatherer population, the Mbuti pygmies of the Congo, also ate large amounts of honey at the time they were studied:
The Mbuti of the Congo forests eat large amounts of honey. According to Ichikawa (1981), honey is their favorite food. At times, during the rainy season up to 80% of the calories in their diet come from honey.
They did not exhibit obesity or insulin resistance.
The Kuna of Panama are interesting because they generally have a non-industrialized diet and lifestyle, except that they obtain white sugar and a few sugar-containing foods via trade. Their diet is 65 percent carbohydrate and 17 percent sugar (95 g/d), according to calculations I did based on dietary intake data. That is more sugar than the average American currently consumes, although 62 percent of their sugar intake is from fruit. The Kuna are lean and have excellent cardiovascular health.
This does not exonerate sugar by any means, but it does suggest that it is unlikely to be the primary cause of obesity, metabolic disease, and cardiovascular disease.
22. During the Cuban economic crisis, sugar intake rose, calorie intake declined, and obesity plummeted
From 1989 through about 1995, the Cuban economy collapsed. Calorie intake declined, fat intake declined, and the diet became very rich in sugar and other refined carbohydrates:
By 1993, carbohydrate, fat, and protein contributed 77 percent, 13 percent, and 10 percent of total energy, respectively, whereas in 1980 their respective contributions were 65 percent, 20 percent, and 15 percent (8). The primary sources of energy during the crisis were sugar cane and rice.
Sugar intake “rose to 28% of total energy intake“. A shortage of gasoline meant that people began walking and riding bicycles more, such that total physical activity increased. During this time, the prevalence of obesity decreased by about half, then eventually rebounded as the crisis resolved and the diet went back to normal. The prevalence of underweight only increased slightly during the crisis, indicating that the decline in obesity was not due to widespread starvation.
23. Cultures with very high intakes of carbohydrate tend to be lean, even if the carbohydrate is white rice
The traditional diet of most agricultural societies around the world is high in carbohydrate and low in fat. Many of these cultures have been studied, and they are typically lean with low rates of diabetes and cardiovascular disease, even if they have abundant food. The book Western Diseases: Their Emergence and Prevention contains many such examples.
In 1990, Staffan Lindeberg, MD, PhD conducted a detailed survey of the diet, lifestyle, and health of the residents of Kitava, a Melanesian island scarcely touched by industrialization. He found a diet based on starchy plant foods (African yam, sweet potato, taro, cassava), fruit, vegetables, seafood, and coconut, with 69 percent of calories coming from carbohydrate (compared to ~49% in the US currently). Despite food abundance, none of the 247 Kitavans Lindeberg examined had obesity or diabetes, even in middle age and older. They had no sign of cardiovascular disease and were unfamiliar with its symptoms. Here is a photo of a Kitavan man being examined by Lindeberg (courtesy of Staffan Lindeberg):

The book Western Diseases: Their Emergence and Prevention contains historical data on the Japanese diet between 1950 and 1975, a time during which obesity was even more rare in Japan than it is today (p. 338). In 1975, long after postwar food shortages were over, the Japanese diet was 62 percent carbohydrate, mostly from white rice. The Japanese ate it daily as their primary staple food, and still do, yet the prevalence of overweight and obesity were, and continue to be, lower than any other industrialized nation. People who emigrate from Japan to the US and begin eating a diet lower in white rice and higher in fat become much heavier, demonstrating that their leanness is not just genetic.
Cross-country comparisons show that nations with carbohydrate-focused diets tend to have the lowest obesity rates, while nations that eat a similar amount of carbohydrate and fat have the highest obesity rates (reproduced from this post with permission from Cian Foley):
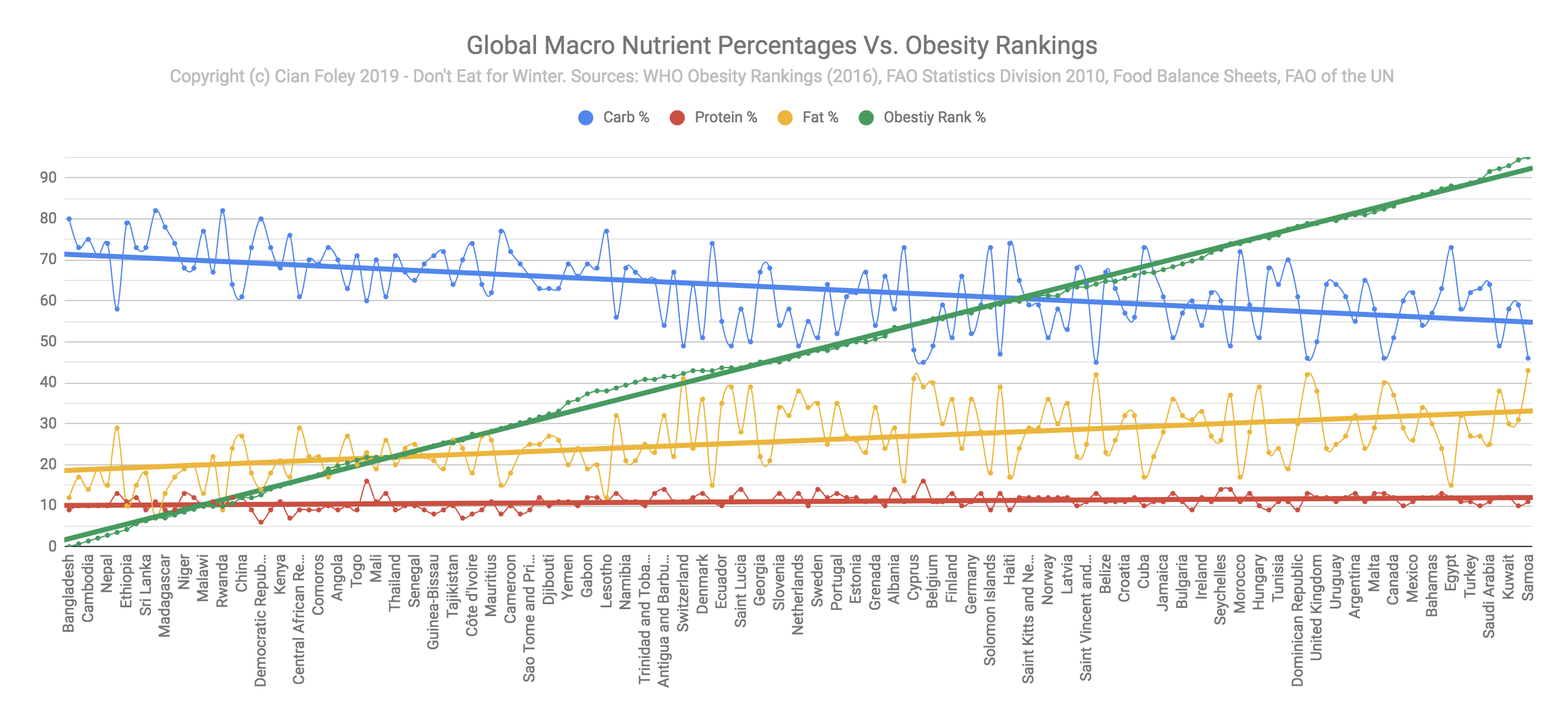
There are certainly explanations for this besides macronutrients– such as poverty– but at the very least this shows that carbohydrate is not an overriding driver of fat gain.
24. In human randomized controlled trials, sugar is only fattening by virtue of its calories
A meta-analysis of 30 randomized controlled trials reported that increasing sugar intake does not increase body weight unless total calorie intake increases. Importantly, calorie intake does often increase when people increase their consumption of sugar-sweetened beverages, so sugar-sweetened beverages aren’t off the hook.
Fructose is half of table sugar (sucrose). A meta-analysis of 31 fructose feeding trials concluded that “Fructose does not seem to cause weight gain when it is substituted for other carbohydrates in diets providing similar calories. Free fructose at high doses that provided excess calories modestly increased body weight, an effect that may be due to the extra calories rather than the fructose.”
25. In randomized controlled trials, low-carbohydrate and low-fat diets yield similar (unimpressive) long-term weight loss results
In real-life conditions, low-fat and low-carbohydrate diets both produce modest weight loss in the average person, usually less than 17 pounds at the 12 month mark. Most meta-analyses (studies of studies) report that weight loss differences between the two diets are “minimal” at 12 months, although low-carbohydrate diets usually fare better up to 6 months. The differences in weight loss between individuals on the same diet are much larger than the differences between diets.
People often dismiss these findings because the diets weren’t lower in carbohydrate. This is a valid critique but it also applies to the low-fat diets used in these studies, which were typically not very low in fat. Also, the fact that people usually don’t restrict carbohydrate or fat as much as instructed shows that most people find the diets hard to sustain in real life.
26. Meta-analyses of ketogenic diet studies
In a quick literature search, I found one meta-analysis of ketogenic diet studies, which reports long-term weight loss after 12 or more months. The ketogenic diets caused 2 pounds (0.9 kg) more weight loss than the low-fat diets.
27. Adherence to ketogenic diets tends to be mediocre
A detailed review of the ketogenic diet literature concluded:
People who are left to their own devices generally do not adhere well to the diet. They typically start strong, but have a hard time adhering to the diet after 1-3 months. Carb intake increases and ketone levels drop.
I have little doubt that better adherence would lead to greater weight loss, but this is true of any weight loss diet.
28. The Virta Health study
The Virta Health study is a non-randomized, controlled trial that reported outcomes for people with type 2 (common) diabetes who followed a ketogenic diet with intensive diet and medical support, vs. care as usual. At one year, participants in the ketogenic diet arm lost 30 pounds (14 kg), lowered their average blood glucose substantially, and greatly reduced their need for diabetes medications other than metformin. Metabolic and cardiovascular risk markers, and the low adverse event rate, suggest that the diet was safe over this period of time. Two-year outcomes suggest that people had begun rebounding back to their original weights and health, but maintenance was still good by the standards of diet interventions.
The Virta Health study reports findings that are quite a bit more impressive than other studies of low-carbohydrate and ketogenic diets. Here are a few reasons why that might be the case:
- Non-randomized trials like the Virta Health study tend to report better results than randomized trials because people self-select into the group they prefer.
- Participants may have been highly motivated because they had type 2 diabetes and the Virta Health program is expensive.
- The Virta Health program provides intensive support to help people adhere to the diet, so adherence was better than in most studies.
29. Eating sugar does not impair weight loss on calorie-reduced diets
I am aware of three weight loss studies that have compared the weight loss response in people eating different amounts of sugar. All three reported that sugar had no impact on weight loss in the context of calorie restriction. One of the studies compared a diet that was 43 percent sugar (sucrose) to one that was 4 percent sugar. Both diets caused the same amount of weight and fat loss over 6 weeks.
Diabetes
30. Weight loss greatly reduces the risk of developing type 2 (common) diabetes
This was conclusively demonstrated by several large, multi-year, randomized controlled trials with actual diabetes diagnoses as the endpoint. One of these trials was the Diabetes Prevention Program study, in which modest weight loss with a calorie-restricted low-fat diet and exercise caused a 58% reduction in the risk of developing diabetes over 2.8 years in 3,234 people with prediabetes. The benefit was largely explained by weight loss, and it was still detectable 15 years later. The diet focused on fat and calorie reduction and was not low in carbohydrate or sugar.
At least four other randomized controlled trials, conducted in four different countries, in people of different races and ethnicities, produced very similar results.
31. Reducing fat intake, without reducing carbohydrate intake, causes weight loss and reduces type 2 (common) diabetes risk
The drug orlistat reduces the absorption of dietary fat in the gut by about 30 percent, without impacting carbohydrate absorption. Large, multi-year placebo-controlled randomized trials have shown that orlistat causes weight loss and reduces the risk of developing type 2 diabetes by 37 percent. This demonstrates that weight loss and protection against diabetes can occur as a result of reducing fat intake, without reducing carbohydrate intake. Orlistat is a FDA-approved weight loss drug.
32. Exercise substantially reduces the risk of developing type 2 (common) diabetes
This was conclusively demonstrated by the Da Qing trial, a 6-year randomized controlled trial. The trial reported that increasing exercise reduces the risk of progressing from prediabetes to diabetes by 46 percent. A second randomized controlled trial reported that two years of supervised aerobic exercise reduced the risk of developing type 2 diabetes by 72 percent, and resistance training reduced the risk by 65 percent. The fact that the exercise was supervised is important– it means the participants actually did it.
33. Weight loss via a temporary very-low-calorie diet durably puts type 2 (common) diabetes into remission
This was conclusively demonstrated by large, a one-year randomized controlled trial in people with type 2 diabetes. The intervention was 3-5 months of a very-low-calorie diet (825–853 kcal/day of a beverage, 59% C, 13% F, 26% P) followed by diet/lifestyle support for the remainder of the year. This caused diabetes remission in 46 percent of people. In other words, 46 percent of people were able to eat normally again and go off medication without experiencing diabetic blood sugar levels for months after the end of the low-calorie diet. Among those who lost the most weight, 86 percent went into remission.
Insulin and insulin resistance
34. Insulin resistance predicts a variety of age-related diseases
In 2001, Gerald Reaven’s group published a very simple but powerful study. They directly measured insulin sensitivity in 147 lean and overweight middle-aged people and waited 6 years to see who got sick, who died, and who didn’t. In the third of the group that was the most insulin resistant, 36 percent developed a health condition or died over the next 6 years, while in the third of the group with the least insulin resistance, none developed a health condition or died. The health conditions that occurred in the insulin-resistant group included high blood pressure, coronary heart disease, stroke, cancer, type 2 diabetes, and death.
35. People with higher insulin levels don’t gain more weight over time than people with lower insulin levels
Many studies have measured insulin levels in an attempt to predict who will gain weight and who will not. Some studies have measured fasting insulin levels, others carbohydrate-stimulated insulin levels. These studies were collected together in a review paper. The research overall suggests that there is no reliable connection between current insulin level and future weight gain. Of the 22 studies included in the review, 5 reported that higher insulin is correlated with more weight gain, 9 reported no correlation between the two, and 8 reported that higher insulin is correlated with less weight gain.
36. Weight loss increases insulin sensitivity, regardless of whether it’s achieved by a low-fat or low-carbohydrate diet
This was demonstrated by a randomized controlled trial in 2009. The low-fat (60% carbohydrate) and low-carbohydrate (20% carbohydrate) diet groups each cut about 500 calories per day and both lost about 9 pounds (4 kg) of body fat over 8 weeks. At the end, insulin sensitivity improved, with no significant difference between groups. Importantly, this study used a gold-standard method for measuring insulin sensitivity, making this result more informative than the vast majority of other low-fat vs. low-carb studies, which tend to indirectly estimate insulin sensitivity using the less reliable HOMA method.
This finding is echoed by an earlier randomized controlled trial in which a 21-day low-fat vs. low-carbohydrate diet, without weight loss, yielded no differences in insulin sensitivity in people with mild type 2 diabetes. This trial also used a gold-standard measure of insulin sensitivity.
37. Insulin resistance is “an appropriate response to nutrient excess“
Exposing cells and tissues to excess energy causes insulin resistance, regardless of whether the excess energy comes from carbohydrate (glucose), fat (fatty acids), or protein (amino acids). This probably happens because excess energy is toxic to cells, so cells turn down their insulin responsiveness in order to take in less energy.
Nutrient excess causes insulin resistance in intact humans as well. Temporarily increasing circulating levels of fat, carbohydrate, or protein in human volunteers markedly impairs their insulin sensitivity.
38. When fat cells “fill up” due to fat gain, energy spills over onto other tissues, causing insulin resistance
As fat tissue expands, fat cells become less effective at taking up fat, especially after meals, and this fat spills over onto other tissues. Depending on underlying genetic factors, different people have different “personal fat thresholds” at which fat gain causes their fat tissue becomes less effective at trapping fat, resulting in insulin resistance and often diabetes.
39. The impact of foods on blood glucose and insulin has little do with how filling they are
This was most clearly demonstrated by a study that fed people the same number of calories from 38 common foods, including bread, potatoes, beans, meat, fruit, peanuts, candy, and pastries, and measured fullness ratings for two hours. Fullness was unrelated to blood glucose responses, and weakly related to insulin responses (higher insulin release correlated with greater fullness).
In contrast, simple food properties like calorie density, protein content, and palatability were strongly related to fullness.
40. Insulin resistance is caused (in large part) by exceeding the unique storage capacity of your own fat tissue
Studies have examined the genetics of insulin resistance, giving us insight into the biological pathways that underlie insulin resistance in the general population. These studies tend to find that gene variants that reduce the storage capacity of fat tissue are associated with insulin resistance.
People with different genetic backgrounds tend to develop diabetes at different levels of body fatness. For example, people of South Asian descent do not need to gain as much fat to develop diabetes as people of European and West African descent. Together, this suggests that each person has a “personal fat threshold“, and if they gain fat beyond that threshold, their fat tissue is no longer able to contain fat effectively, it spills over onto other tissues, leading to insulin resistance and a high risk of diabetes.
41. Mendelian randomization studies on the impact of insulin level on body fatness
Mendelian randomization is a genetic technique that can help to understand the relationship between risk factors and health conditions. Several such studies have examined the relationship between insulin secretion and body fatness. The first two reported no effect of insulin secretion or fasting insulin level on body fatness. The third study reported that insulin secretion increases body fatness, but that this mechanism only accounts for 1-10 percent of between-person differences in body fatness.
Thus, all Mendelian randomization studies to date suggest that the impact of insulin on body fatness is either small or nonexistent.
42. Of the three published studies funded by Taubes’s organization the Nutrition Science Initiative (NuSI), at least two, and possibly all three, refuted the carbohydrate-insulin hypothesis
The first study was a tightly controlled metabolic ward study reporting that metabolic rate slightly and transiently increased, but fat loss actually slowed, on a very-low-carbohydrate ketogenic diet (2% sugar), compared with a high-sugar (25%) higher-carbohydrate diet of equal calories. This study also reported that the very-low-carbohydrate diet did not increase total energy levels in the bloodstream, as claimed by Taubes’s model.
The second study was a one-year randomized controlled diet trial (DIETFITS) reporting that a low-fat vs. low-carbohydrate diet led to the same degree of weight loss and the same metabolic rate after one year, and baseline insulin secretion did not predict who did better on which diet.
The third study reported that a very-low-carbohydrate diet led to higher calorie expenditure than a low-fat diet in people maintaining weight loss. However, a reanalysis of the raw data suggests that the effect may be an artifact of faulty data.
Exercise
43. Exercise tends to cause weight and fat loss in people with excess body fat
This has been demonstrated by dozens of randomized controlled trials, although the findings are not always consistent across individuals or trials, probably because many trials use modest exercise interventions and people adhere to them poorly. Meta-analyses (studies of studies) tend to report that exercise causes weight and fat loss, including the most recent meta-analysis of 36 studies.
Greater volume of exercise, particularly when supervised to ensure compliance, causes greater weight and fat loss. In one randomized controlled trial that assigned overweight volunteers to different exercise amounts, greater amounts led to more body fat loss, with the greatest amount leading to a loss of 12 lbs of body fat over eight months relative to a group that did not exercise (175 min a week on a cycle trainer, treadmill, or elliptical).
Thanks to Adam Tzur of Sci-Fit for references.
44. Exercise increases insulin sensitivity
This has been demonstrated by many randomized controlled trials. Here are a few review papers of the evidence.
45. Sedentary behavior causes insulin resistance
Athletes have high insulin sensitivity, yet ten days of inactivity markedly reduces it. Five days of bed rest markedly increases insulin resistance in non-athletes as well. Reducing daily walking time also promotes insulin resistance in only two weeks.
Food reward
46. Fat and carbohydrate both cause dopamine release and increase eating drive, particularly when combined
The gut senses fat, carbohydrate, and protein, and this causes dopamine release in the brain that stimulates motivation and learning. This has been extensively documented in animals, and in humans it has been replicated for fat and carbohydrate.
The combination of carbohydrate and fat causes greater activity in dopamine-responsive brain regions, and greater eating drive, than either alone. To visualize this yourself, imagine how much you would want to eat regular ice cream, vs. fat-free ice cream, vs. unsweetened ice-cream.
47. The most commonly/intensely craved foods combine carbohydrate and fat together, while foods that are high in sugar or fat alone are less commonly craved
This should be common sense (think of ice cream without the sugar or fat), but it has been extensively supported by research. Foods rich in fat and carbohydrate together stimulate eating drive more than either alone, measured both by brain activity and people’s willingness to pay for the foods.
Studies on cravings and food addiction have found that foods combining carbohydrate and fat are the most likely to trigger cravings and addiction-like behaviors, including savory foods that contain no sugar. The most commonly craved food is chocolate. Foods that are only high in sugar or fat can also trigger cravings, but it’s less common.
48. We eat more food when it tastes better
This is common sense, but here are some references to scientific studies just in case anyone doubts it.
Rigor
49. Calling for more rigor in science does not necessarily make a person rigorous
Science has flaws at many levels that make it less efficient and effective than it could be. However, using the concept of rigor selectively to criticize findings you don’t like is not rigorous.
Those who genuinely care about scientific rigor should be promoting the most effective tools we have for increasing it: Study preregistration, open data, proper application of statistics, conflict of interest regulations and disclosures, replication, meta-analysis, and Registered Reports. They should also be promoting the organizations that develop and advance these tools like the Center for Open Science. The most effective tools for increasing the rigor of science are coming from within science itself– designed by people who understand how science works. Non-scientists can help by learning about these efforts, promoting them, and pushing for their adoption.
50. Expert reviews of Taubes’s writing have uncovered extensive misuse of evidence
Gary Taubes has an extensive history of misrepresenting source material to benefit his narrative. The most detailed fact checks of his books were written by Seth Yoder at The Science of Nutrition, and the results are frankly disturbing. My own review of The Case Against Sugar also uncovered evidence misuse and a general unwillingness to cite relevant scientific evidence and apply logic appropriately.
51. The Nutrition Science Initiative (NuSI), with Gary Taubes at its helm, attempted to interfere with the research that it funded once unfavorable results came in
Initially, NuSI assured researchers that it would have “no control over research design, conduct, or reporting”, which was essential due to Taubes’s conflicts of interest. NuSI asked lead researcher Kevin Hall to communicate this to me, which he did in 2012 and I documented on my website. Once the first results came in undermining the carbohydrate-insulin hypothesis, NuSI broke its agreement and began interfering with the scientific process, eventually causing the resignation of lead researcher Kevin Hall. Hall concluded that “Potentially conflicted funders should not be involved in the conduct, analysis, or reporting of research. NuSI’s meddling in the EBC study violated both the final contract terms and our verbal agreement at the outset of the study.”
Miscellaneous
52. The most fattening diet in animals and humans is human junk food, and its fattening effect cannot be explained by carbohydrate or fat alone
In rodents, the most fattening diet ever tested is the “cafeteria diet”, which consists of a buffet of calorie-dense, tasty human foods like condensed milk, chocolate chip cookies, salami, cheese, banana, marshmallows, milk chocolate, and peanut butter. When rodents are given a choice between the cafeteria diet and their normal low-fat rodent pellets, not surprisingly they gorge on the human foods and virtually ignore the boring pellets. Other species quickly grow fat on calorie-dense human food as well, including raccoons and monkeys. Humans with ready access to a variety of calorie-dense, tasty foods also overeat and gain weight rapidly.
In rodents, diets that are only high in fat or only high in refined carbohydrate (including sugar) are not nearly as fattening as the cafeteria diet, demonstrating that neither fat nor refined carbohydrate alone can explain its full fattening effect. Similar findings are observed in humans, where increasing sugar intake alone isn’t nearly as fattening as the cafeteria diet.
53. Obesity has a strong genetic component
This has been demonstrated by many different studies in humans. A meta-analysis (study of studies) of 115 such studies concluded that 75 percent of the difference in body fatness (BMI) between people is due to genetic differences. Note: this is perfectly compatible with the idea that diet and lifestyle changes are responsible for the high prevalence of obesity in the 21st century; these studies describe the genetic component of differences in body fatness between people in a given environment. If we all live in a fattening environment, those of us who are genetically susceptible to obesity will develop it. Without a fattening environment, these genetic differences matter less and few people develop obesity.
54. Synthesis of fat from carbohydrate is negligible in humans eating typical Western diets
The scientific term for this is de novo lipogenesis, and although the pathway exists in humans it only converts a tiny fraction of ingested carbohydrate into fat under realistic circumstances (typically less than 1 gram per day). In contrast, most people consume 50-200 grams of fat from the diet. See this review paper for the evidence, or the physiology textbook Metabolic Regulation, pages 200-201.
55. Partially suppressing the release of fat from fat cells (lipolysis) does not cause fat gain, hunger, or a slower metabolic rate
A drug called acipimox inhibits lipolysis, or the release of fatty acids from fat cells, mimicking the effect of insulin. As a consequence, free fatty acid levels in circulation decline (this is a key circulating fuel in the bloodstream). The carbohydrate-insulin hypothesis predicts that this should lead to fat gain, hunger, and a reduced metabolic rate. A 6-month placebo-controlled randomized controlled trial of acipimox showed that the drug reduces circulating free fatty acid levels by 38 percent but has no impact on body composition, calorie intake, or metabolic rate.
56. The fat tissue of people with obesity releases fat at a greater rate than that of lean people, not a slower rate
The most informative study to date on this found that the greater a person’s fat mass, the higher the total rate of fat (free fatty acid) release. Thus, nothing is “trapping” fat inside the fat cells of people with obesity– to the contrary, they release fat from their fat tissue at a greater rate than lean people.